Implantable drug-eluting device: localised drug delivery for non-resectable pancreatic cancer
Research project update
This multidisciplinary research collaboration has brought together leaders in Cancer Biology and Therapeutics, Oncology, and Biomedical Material Science in a project that has developed a novel drug delivery device for the treatment of locally advanced, non-resectable pancreatic cancer.
Currently, the survival of pancreatic cancer patients is poor due to two main factors; late-stage diagnosis leading to advanced inoperable tumours and the dense, scar-like tissue that surrounds pancreatic tumours making them resistant to systemically administered chemotherapy. The team aim to overcome the above challenges by delivering the therapy directly to the tumour via a biodegradable implantable device, whereby it acts to penetrate the stiff tissue layer that protects the pancreatic tumour and confining the distribution of drugs to the tumour, leaving healthy tissue untouched. Altogether this means reduced drug dose is required to achieve tumour shrinkage and toxic side effects are minimised.
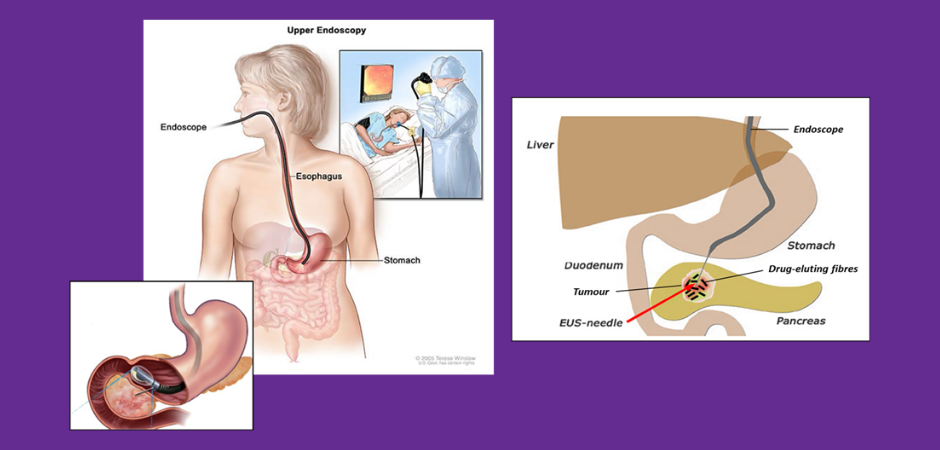
To date the team has developed a degradable drug eluting polymeric structure, about the size of a grain of rice, that is suitable for implantation directly into the tumour via endoscopic ultrasound-guided fine needle injection (EUS-FNI). The team have tested the device loaded with a combination of chemotherapeutic drugs including gemcitabine and paclitaxel, and more recently, the FOLFIRINOX regimen. Results show the drugs are localised to the tumour and not dispersed throughout the body, compared with intravenous (IV) drug administration. Importantly, treatment with drug loaded implants reduced tumour volume or completely eradicated tumours in 66% of mice while only 20% of mice responded when the same drugs were administered IV.
They also examined the ability of the implant to reduce side effects, by monitoring the way in which fluorescently labelled drugs behaved within the body compared to standard IV delivery. Drug delivery via the implant showed a dramatic confinement of the labelled drug to the site of implant, while IV administration resulted in drug distributing throughout the body. This is exciting, as the development of this delivery method will lead to a smaller therapeutic dose, which in turn will lead to fewer side effects, better tolerated therapy and in the best-case scenario, reducing the size of the tumor to an operatable status.
To ensure rapid translation of the research findings to patients the team:
- has created a novel drug delivery platform from all FDA-approved materials,
- has assessed safety and efficacy in clinically relevant pancreatic cancer -mouse models and
- Prepared a physiologically relevant pancreatic tumour model for testing new drugs in vitro: Collagen embedded tumour spheroid model, allowing more accurate and high-throughput preclinical testing to be performed in vitro.
- will assess the safety of the implant containing therapeutic drug concentrations in a porcine model (delayed by COVID-19).
- Prepared a first-in human phase I clinical trial protocol for locally advanced pancreatic cancer. The protocol has been presented as an early-stage research concept at the AGITG annual scientific meeting and 2021 AusBiotech Early- stage Investment Forum symposium for expert feedback on the study design and feasibility.
This project is supported jointly by Pancare and Cancer Australia’s Priority-driven Collaborative Cancer Research Scheme (PdCCRS).
Read more . . .






